Albumin is a natural material that covers nearly 60 per cent of the human proteome and is utilised as a buffering protein that helps transport various xenobiotics in the body. Pharmaceutically, it is used for the treatment of hypovolemia in patients with chronic conditions. Albuminbased Abraxane® (paclitaxel-albumin nanoparticles) is a billion-dollar drug for treating various cancer conditions. It helped to provide an organic solvent-free formulation of a hydrophobic drug (paclitaxel). There has been an exponential rise in the research to explore various applications of albumin as a biomaterial for therapeutic and formulation development applications. As a pharmaceutical biomaterial, albumin can be moulded to form nanoparticles, hydrogels, nanofibers, and films. This article highlights key aspects, such as the synthesis and application of albumin as a pharmaceutical excipient in developing dosage forms.

Albumin is a globular protein soluble in water and coagulable by heat. It is found in egg white, blood serum, milk, and other animal and plant tissues and fluids. Human Serum Albumin (HSA) is the most abundant protein in human plasma. The use of colloidal albumin for therapeutic purposes dates back more than 50 years. Purified albumin solution is used clinically in the treatment of chronic illnesses dealing with hypovolemia (5 per cent or 25 per cent w/v). The extraction of albumin has been optimised and perfected over time. Being a natural xenobiotic career in the body, albumin (as a biomaterial) was used to synthesise nanoparticles. These are tiny particles (~200 nm size) for nanomedicine applications. HSA shows reversible binding capabilities with a range of substances, such as fatty acids, hormones, and metal ions. This makes it an ideal biomaterial for developing novel drug delivery and biomedical imaging therapeutics. This subsection narrates the uses of albumin materials in developing nanomedicines and semi-solid dosage forms.
Albumin nanoparticles are typically prepared using various methods, including desolvation, self-assembly, thermal gelation, spray-drying, emulsification, nanoparticle albumin-bound (Nab) technology, and pH coacervation. Each of these methods has its advantages and disadvantages, and the choice of method depends on the specific requirements of the drug delivery system. The desolvation method, for example, involves the removal of solvent from a solution, causing the albumin to precipitate and form nanoparticles. On the other hand, the self-assembly method relies on the natural tendency of albumin molecules to aggregate into nanoparticles under certain physical or chemical stress conditions.
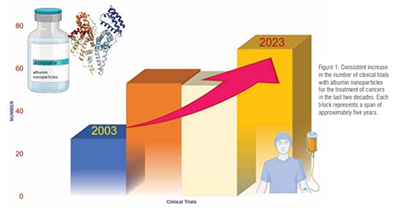
One of the most well-known commercialised albumin nanoparticles is Abraxane®, which uses a technology known as Nab technology. This technology takes advantage of the natural properties of albumin and its accumulation in tumours. The drug paclitaxel is bound to albumin and delivered to tumours without the use of toxic solvents. Abraxane® binds to the albumin receptor of the endothelial cells, activating a process called caveolae-mediated transcytosis, which enhances the delivery of the drug to the tumour. Currently,>200 clinical trials are being conducted to explore the use of Abraxane® as a single or combination therapy to develop various therapeutic options (Figure 1). Moreover, many other particles are being produced using albumin nanoparticles as a delivery system, as shown in Table 1.
Looking at the success of albumin as a nano delivery system, this biomaterial was used to develop theranostic nanomedicines for various anticancer drugs. Following are some examples:
a) Photo thermal for melanoma: A newly synthesised near-infrared dye IR-817 was combined with bovine serum albumin (BSA) to create BSA@IR-817 nanoparticles (120 to 200 nm). These nanoparticles were assessed for their fluorescence imaging (due to IR-817 dye) and photothermal (due near IR 808 irradiation) therapy potential against melanoma.
b) Photothermal therapy combined with MRI imaging of cancer: A selfassembly method of albumins with small molecules was used to encapsulate IR780 and super paramagnetic iron oxide (SPIO) nanoparticle, that forms IR780@BSA@SPIO nanoparticles. They provided NIR-II and MRI dual-mode imaging, that can accurately find cancer and guide photothermal therapy to eliminate tumours.
c) Albumin-coated or functionalised quantum dots were developed for bioimaging applications. These albuminbased nanoparticle designs were evaluated under in vitro or in vivo conditions. However, more data related to pharmacokinetics, biodistribution, dose safety and toxicity are required for their clinical testing.
Besides, microparticle designs like albumin microbubbles have been explored for ultrasound-mediated diagnostic applications. These microbubbles are typically 0.5–10 μm in size, which makes it easier for medication delivery mechanisms to navigate the body. Microbubbles made of albumin are effective vehicles for delivering photodynamic agents—drugs used in advanced anti-cancer therapy that avoid the side effects of chemo- and radiation therapy. Moreover, microbubbles have been utilised as contrast agents during ultrasound procedures. However, there has been a recent focus on exploring their potential as drug-delivery systems that respond to specific stimuli. The original microbubbles, known as Albunex, which consisted of a protein shell made from human serum albumin, were developed and successfully adopted in clinical practice in 1993. As a final observation, albumin microbubbles have shown significant potential in the clinical field, particularly in drug delivery and disease treatment. Their unique properties allow for targeted and efficient delivery of therapeutic agents, making them a promising tool in modern medicine.
From a formulation perspective, several challenges need to be addressed. These include issues with reproducibility, proper characterisation, and biological evaluations. For example, the size and shape of the nanoparticles can affect their behaviour in the body, and these properties can be challenging to control. In addition, the interaction of the nanoparticles with the immune system is not fully understood and can lead to unexpected side effects. Therefore, rigorous studies alongside stringent guidelines for effective and safe nanomedicine development and use are still called for.
Hydrogels are a semisolid dosage form. Pharmaceutical gels are primarily prepared from semi-synthetic or synthetic materials with polysaccharides as their backbone. Examples include methyl cellulose, hydroxyethyl cellulose (HEC), hydroxypropyl methylcellulose (HPMC), and others. Methylcellulose (water soluble) is a cellulose derivative used for bowel irregularity and is available over the counter. All these derivatives have variable water solubility and thickening behaviour. This also makes these materials being explored as suspending agents in the formulation development. Since these materials can produce viscoelastic gels, the cellulose-based systems provide an essential foundation for using sustainable green material for semi-solid formulation development. Significant research has been done to understand the sustainability of green materials for developing semisolid preparations.
Protein is one of the natural building blocks, and it was observed that the albumin-protein solution can produce a transparent hydrogel. Since then, researchers have been creating these albumin hydrogels for a potential real-world application. Over the years, research has been conducted to understand the gelling behaviour of albumin-based or albumin. Chemical crosslinking of albumin or albumin-based gels can be achieved through disulfide cross-linking under acidic pH. Solvents like ethanol and a composite of albumin-succinimidyl succinate-modified polyethylene glycol (PEG) provided gels with injectable behaviour. Using chemical denaturants in gel formation can provide gels, improper for utility due to toxic residues. Alternatively, the physical crosslinking phenomenon of albumin solutions to create hydrogel, is another method that is explored by some researchers, including our lab. The drug-binding nature of albumin combined with the fact that it can become a gel has led people to believe that albumin hydrogels could be a new form of drug delivery system. The hydrogels can be loaded with the drug and then injected into the body, where they slowly release the drug over time. This allows for a more targeted and controlled drug delivery, which can improve its efficacy and reduce side effects.
The formation of albumin hydrogels creates a biomaterial that shows various biological and mechanical properties, making it a promising substance for drug delivery. The unique mechanical properties of the gel allow it to have a range of tensile strengths, leading to its ability to form hydrogels suitable for drug delivery (low strength) and hydrogels that aid in tissue regeneration, providing structural scaffolding for new growth (high strength). Moreover, albumin-based gels can provide a supportive scaffold for new tissue growth. They can also be loaded with growth factors or other bioactive molecules to promote tissue regeneration. It could also be used as a cartilage replacement material. A firm but elastic gel for a knee replacement has the potential to give the knee the cushion that normal cartilage provides that no ceramic or metal material could provide. Albumin hydrogels can potentially have significant uses in medicine and other areas of life. The situation is ever evolving, and innovative ideas and discoveries are made every day. Hopefully, albumin as a biomaterial and therapeutic agent could answer many more questions than we even realise.
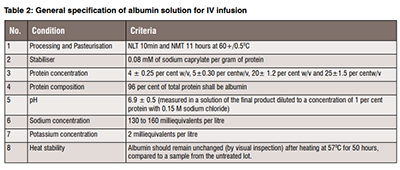
From the excipient point of view, bovine serum albumin (BSA) is the most explored material by various research laboratories. From the clinical perspective, USP supplies detailed monograph data about recombinant human albumin. The monograph supplies details about the formulation of human serum albumin for intravenous use. FDA gives a manufacturing procedure for the fractionation of human serum albumin and a comparison of various products to provide clinical usefulness of albumin solution. The Code of Federal Regulations (CFR) includes information about the fractionation of serum albumin for making albumin solutions in clinical use. Still, the standard chemical signatures of albumin as material necessary to prevent batch-to-batch variation is unclear. However, the data provided by CFR, USP, and FDA (Table 2) can lay the foundation for creating material-specific monographs of albumin for its use in formulation development.
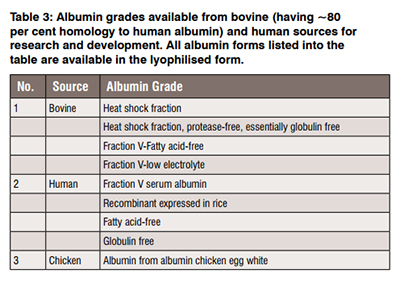
Albumin fractionation from various sources (bovine, human, egg, chicken, and others) dictates the quality of biomaterial isolated. The process of fractiona tion of human albumin was set up nearly 60 years ago and is still the method that is being explored for isolating human albumin for clinical use. Various grades of albumin being synthesised (Table 3) are constantly researched in literature to create advanced controlled delivery formulation.
Each supplier company (like Sigma, Fisher Scientific, Bio World, and others) provides technical specifications of bulk material. However, no standardised criteria or standards are available to avoid inter and intra-company variability for the formulation development. This highlights the potential challenge of envisioning a biological product as a pharmaceutical excipient for formulation development.
Albumin is a pivotal and versatile material in pharmaceutical science, highlighting its significance in diverse dosage forms and drug delivery systems. It is a sustainable green biomaterial for advanced delivery systems. The evolution of albumin-based formulations, exemplified by Abraxane®, has revolutionised cancer treatment, offering a solvent-free platform for hydrophobic drugs and prompting numerous clinical trials exploring its therapeutic potential. The use of albumin in nanomedicine has opened new avenues, with albumin nanoparticles (shown in Table 1) showing promise in drug delivery and potential theranostic systems. Applications such as enhanced imaging and photothermal therapy underscore albumin's diverse roles in advancing medicine. Exploring semi-solid dosage forms, particularly albumin hydrogels, adds another dimension to albumin's pharmaceutical versatility. Albumin hydrogels offer a potential platform for controlled drug delivery and tissue regeneration engineering.
As a pharmaceutical excipient, albumin, mainly bovine serum albumin, is commonly explored in laboratorybased- academic research. Challenges such as the need for standardised criteria to ensure consistency across formulations. Addressing these challenges is crucial for proving albumin as a reliable pharmaceutical excipient. Groundbreaking achievements and ongoing challenges mark Albumin’s journey in pharmaceuticals. As research continues, albumin holds promise not only in cancer treatment but also in diversified applications, pushing the boundaries of drug delivery and formulation development, potentially addressing unmet medical needs, and providing innovative solutions in various domains of medicine.