Biomimetic nanoparticles are emerging as next generation nanotherapeutics deemed to overcome the known limitations of previous generations. Their biological origin and customisable nature can promote the process of therapy personalisation and enable new precise delivery approaches. Albeit affected by yet unmet gaps, biomimetic nanoparticles possess all requisites for a near to come successful clinical application.
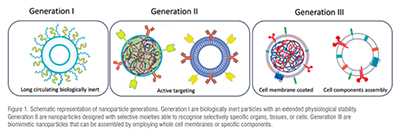
The past two decades have witnessed mind blowing progress in concepts, strategies as well as technologies that are about to revolutionise the nanomedicine landscape. Since the 80s-90s, nanocarriers have been continuously evolving to face the hurdles of delivering drugs more and more precisely to a specific target. Such advances have promoted the rise of innovative therapies for a broad range of diseases. For instance, cancer treatments have benefited from the enhanced ability to localise drug action to a malignant cell neighbouring space leaving the healthy ones nearly untouched. An increased understanding of the multiple barriers and the fate of nanoparticles in the organism fostered new approaches in nanoparticle manufacturing technology leading to new generations of carrier engineering strategies. Today, at least two generations of nanoparticles are being recognised. The first are biologically inert particles able to ensure drug accumulation based on an extended physiological stability. Long-circulating liposomes that have also been the first and one of the few nanocarrier products to reach the market, belong to this class. The second-generation nanoparticles are instead designed to target specific organs, tissues, or cells through moieties, such as lipoproteins or antibodies, that confer high selectivity.
Though engineered to meet specific requirements, nanoparticles can be brutally transformed by surface interactions in the physiological environment. This is a well-recognised yet not fully understood phenomenon named protein corona effect, by which proteins present in the biological fluids bind the nanoparticle surface mutating the nanoparticle native identity into a rather inconstant biological one. This poses numerous challenges, as such a phenomenon, although partially preventable, is unpredictable, being prone to the intrinsic variability of biological systems. Age, sex, health conditions, lifestyle, environment, food, and inner physiology all together contribute to a change in the protein corona properties and the biological identity of the nanoparticle therein. Needless to say, this phenomenon hampers nanoparticle targeting capability and physiological stability.
Nevertheless, the whole scenario seems to be changing since the biomimetic nanoparticles’ first appearance in early 2010s. These conceptually new nano-systems can be considered third-generation nanoparticles (Figure 1). Their main feature is their biological origin, as they are generated from cell membranes or assembled using hybrid biomaterials. These nanoparticles are engineered to recapitulate physiological and target features. This approach is thought to underpin the evasion of the organism engagement rules to ensure higher targeting efficacy and consistency. Albeit in their infancy, these systems may open new and unexplored possibilities in the years ahead.
Hitting a therapeutic target is a rather complicated task, which lays on the intrinsic anatomical and physiological complexity of the organism. Drug targeting is accomplished by ensuring progressive accumulation of the carrier to the site of action through a mix of physical and chemical processes. Stability, diffusivity, and affinity must team up to allow the carrier to successfully engage the target over a required time frame. These three dimensions of drug targeting can exploit passive or active mechanisms by which the nanoparticle can either permeate physiological barriers or recognise specific target features that enhance selectivity and specificity of treatment. To this picture, nanoparticle biomimetics can be accounted as a fourth dimension of drug targeting. In fact, biomimetic nanoparticles can evade the known constraints by paving new avenues. The possibility to adapt and indulge environmental and target features represents a pivotal crossroad that may foster new and modern patient-centred therapeutic approaches. After the advent of RNA nanotherapeutics, which could not have been possible without the development of suitable nanocarriers, biomimetic nanoparticles may represent the next breakthrough as they may allow to circumvent physiological barriers and exploit processes and mechanisms previously inaccessible. Indeed, biomimetic nanoparticles may promote therapeutic effects by triggering biological cascade processes. Drug-free nanocarriers enabling the targeting of so far undruggable pathways may be envisaged in the near future. Research is rapidly moving forward in this field, nourishing the slow but relentless evolution of nanoparticles from simple carriers into precise functional therapeutic systems.
Nanoparticle technology evolution is benefiting from the increased knowledge and understanding of the complexity of biological systems. An example in this sense is the fast-growing research around extracellular vesicles as potential carriers and therapeutic systems that may indeed represent a quantum leap in the progress of medical science. In a way, biomimetic nanoparticles are close relatives to extracellular vesicles as they can share similar basic features and origin as well as an intrinsic versatile and tailorable nature. Such a potentially customisable nature of biomimetic nanoparticles makes them an attractive platform for a broad range of applications. They can be conceived as entities recapitulating all the wanted biological features through specific engineering and assembly of biological materials. Customisation may depend upon specific therapeutic requirements. Similarly to other scientific areas, technologies already exist that can be employed to engineer cells to express specific factors and membrane composition that, through proper manipulation, can be translated to nanoparticles that then will replicate the same features. Therefore, nanoparticle functional personalisation based on disease and patient conditions can be conceived by exploiting the patient’s autologous cells as a source. Thus, next generation nanoparticles are on their way to become novel customisable and precise therapeutic systems.
Recent research trends and clinical evidence suggest that therapy personalisation is a key process that may contribute to a remarkable improvement of therapeutic outcomes. It is today clear that providing the right treatment to the right patient at the right time is an avenue to clinical success. Nevertheless, personalisation requires proper tools for optimal choice of interventions. Several approaches are currently adopted for treatment suitability testing, and some of them are already clinically employed to address issues in the personalisation of interventions. Other emerging strategies rely on a patient’s genetic profiling, health monitoring, or, where feasible, the generation of a patient’s cellular avatars. The latter is a strategy that consists in harvesting the patient’s own cells and, using stem cell technology, producing other cell types. As an example, the organoid technology is already employed in oncology and cystic fibrosis for clinical drug screening to address suitability of treatment. However, such a huge personalisation effort would be in vane if not supported by the development of proper delivery approaches. Biomimetic nanoparticles can be pivotal in such a process allowing to model the delivery strategy upon individual peculiarities.
Beyond the obvious impact of therapy personalisation on the patient’s qualityof- life, especially in chronic conditions, the change is expected to also affect the Pharma industry whose market strategies and investments require rethinking. In part, this is an already ongoing process, as companies started to scale down their production system and develop proper control strategies and enabling technologies. Personalised medicine has already ended the pharma blockbuster era by parting the drug market into smaller defined niches. A big role in this regard is being played by the orphan drug market, which has become a driving force in the development of personalised medicine due to intrinsic unmet needs that demand precise and specific therapeutic approaches and the dedicated and expedited market authorisation procedures promoted by regulatory agencies.
Huge change lies ahead for the industry driven by a digitalisation and the inroads made by artificial intelligence (AI) into R&D and pharmaceutical production. Digitalisation is rapidly gaining ground for several pharmaceutical products and medical devices. In particular, orally inhaled and nasal drug products start taking advantage of advanced electronic devices that facilitate monitoring and control over patient’s self-medication. In general, digitalisation underpins improved performances and patient-centred therapy assessment. In addition, AI has already permeated large areas of fundamental and applied research and is dramatically imposing into clinical research as well. The power of AI algorithms is enabling progress towards an insightful understanding of complex systems, such as living organisms. Today, AI is already employed for patients’ health monitoring and profiling to lay the foundations for therapy personalisation. Indeed, the digitalisation and AI waves can promote the transition to personalisation of therapies and products by enabling the assessment of the best conditions for the precise and customised delivery of treatment. Approaches such as machine learning and deep learning are already employed in R&D to help the development of optimised therapeutic systems.
The above discussed biomimetic nanoparticle peculiarities can affect this transition process. Unfortunately, a setback to this fast-moving scenario is that regulatory bodies are playing catch-up with scientific progress. Such a situation is producing an understandable yet arguable bottleneck to the translation of novel therapeutic strategies to the clinic. When dealing with systems of biological origin such a gap broadens, owing to the lack of information on their fate and toxicity. Aspects that restrain the rush to the clinic deal with instability concerns in the first place, as nanoparticle size, composition, and physical–chemical properties change significantly according to source and preparation methods. Moreover, largescale production is today still far from being achieved and significant endeavours are required to address purity, component as well as drugs loading, and process yield issues. Linked to the above considerations, safety of biomimetic nanoparticles remains unclear in humans and additional efforts are demanded to assess their biochemical properties, pharmacodynamics, and pharmacokinetics.
Undoubtedly, nanoparticle biomimetics can unleash the huge potential of nanotherapeutics, albeit their journey as enabling tools to precision and personalised medicine has just begun. Indeed, biomimetic nanoparticles have the power to contribute to the transformation involving R&D and production environments even thanks to digitalisation and AI support. Unexplored scenarios are opening ahead with likely unprecedented clinical impact. However, barriers exist that can considerably slow down this yet irreversible process. Although biomimetic nanoparticles are still behind the establishment of proper clinical settings, the progress made in the development and approval of new cell and gene therapies indicate that reaching the clinic for biomimetic nanoparticles is only a matter of time.